Organic chemistry
conjugated systems (sp2 hybrids)
from
CH2=CH-CH=H2 + HBr (1,2 or 1,4 addition)
___________________
Kinetic product
CH2+-CH=CH-CH3 to CH2Br-CH=CH-CH3 (more stable)
or
Thermodynamic product
CH2=CH-CH+-CH3 to CH2=CH-CHBr-CH3 (reacts faster)
Diels-Alder reaction
between conjugated diene and substituted alkene (dienophile) to form a
cyclohexene
MO Energy Diagram (antibonding vs. bonding)
higher energy states can only be reached with activation energy added to
system
*bottom to top pi 1, pi 2, pi 3, pi 4
Ruckel's rule
continuous p orbital is planar, non-continuous (non-aromatic) is non-planar
Hund's rule
polygon rule
electrophilic aromatic substitution
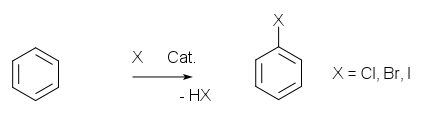
In the first step of the
reaction mechanism for this reaction, the electron-rich aromatic ring which
in the simplest case is
benzene
attacks the electrophile A. This leads to the formation of a
positively-charged cyclohexadienyl
cation, also known as an
arenium
ion. This
carbocation is unstable, owing both to the positive charge on the molecule
and to the temporary loss of
aromaticity. However, the cyclohexadienyl cation is partially stabilized by
resonance, which allows the positive charge to be distributed over three
carbon atoms.
In the second stage of the reaction, a
Lewis base B donates electrons to the hydrogen atom at the point of
electrophilic attack, and the electrons shared by the hydrogen return to the
pi system, restoring aromaticity.
An electrophilic substitution reaction on benzene does not always result in
monosubstitution. While electrophilic substituents usually withdraw electrons
from the aromatic ring and thus deactivate it against further reaction, a
sufficiently strong electrophile can perform a second or even a third
substitution. This is especially the case with the use of
catalysts.
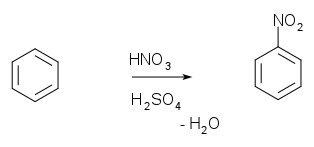
aromatic nitration: nitric acid + sulfuric acid = NO2+
(nitronium ion)
rxn with benzene produces nitrobenzene
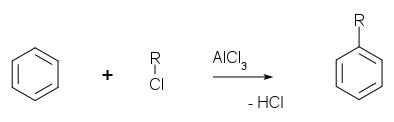
Friedel-Crafts reaction
The
Friedel-Crafts reaction exists as an
acylation
and an
alkylation with acyl halides or
alkyl halides as reactants.
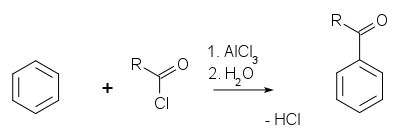
The catalyst is most typically
aluminium trichloride, but almost any strong
Lewis acid can be used. In Friedel-Crafts acylation, a full measure of
aluminium trichloride must be used, as opposed to a catalytic amount.
organic acids: constants
resonant effects:
inductive effects: electron withdrawing groups make an alcohol (and phenol) a
stronger acid by stabilizing the conjugate base (alkoxide)
diols: cis-1,2 from hydroxylation of an alkene with OsO4 followed by reduction
with NaHSO3
trans-1,2 from acid catalyzed (peroxy acids) hydrolysis of epoxides

from aldehydes and ketones
from carbonyl

Sodium borohydride is
a milder reducing agent, can be used in aqueous solution. It converts
selectively aldehydes and ketones the corresponding alcohols
in the manufacture of
pharmaceuticals and other fine chemicals.
It will not react with esters, amides, or carboxylic acids, the more powerful
reducing agent lithium aluminum hydride(LAH) is used to reduce these compounds.
LAH is the more powerful reducing agent than sodium borohydride due to the
weaker Al-H bond compared to the B-H bond. The reactivity of sodium borohydride
can be modified by addition of iodine or methanol in BH3-THF
to reduce esters into the corresponding alcohols like the reaction of benzyl
benzoate to benzyl alcohol. Sodium borohydride is used as a hydrogen source for
fuel cell systems and a foaming agent for rubbers. Sodium cyanoborohydride
converts certain alcohol groups to methylene groups. Sodium Cyanoborohydride is
used as a selective amination reductant. It converts aldehydes (chemoselective),
ketones (stereoselective) to the corresponding alcohols
in the manufacture of
pharmaceuticals and other fine chemicals.






